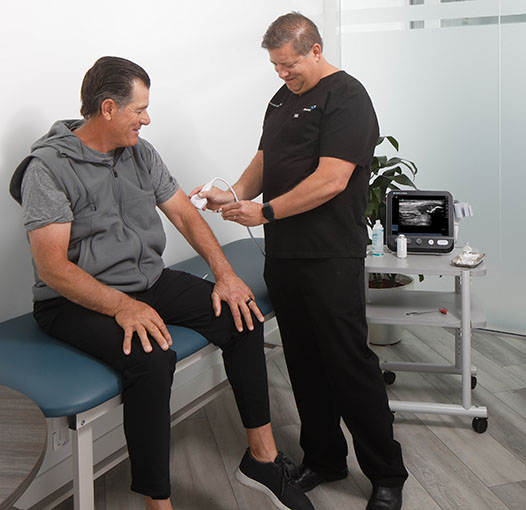
Our team of doctors are committed to making chronic joint pain a thing of the past. We start by getting to know you and your medical history, followed by a few simple tests to determine the best course of treatment for you.




Once you have begun your recommended course of treatment, our medical team will follow your progress with regular visits to address any further concerns you may have pertaining to recovery and daily activities.
The StemX process is transparent and hassle-free with regular checkups on your comfort and progress. Schedule a free consultation to see how we can help.


Evaluation
Our team will begin by assessing your health history, current medications, and prior treatments to determine the best treatment options for you.
Imaging
We use/utilize MRI and ultrasound to help us evaluate the damage and degeneration in the affected joint.
Treatment
Treatment options for your condition will be explained in detail by your doctor along with pre and post-treatment instructions.Other Services:
Outside of insurance covers, we offer the following therapies. Our clinicians will go over these treatment plans with you in detail after your evaluation.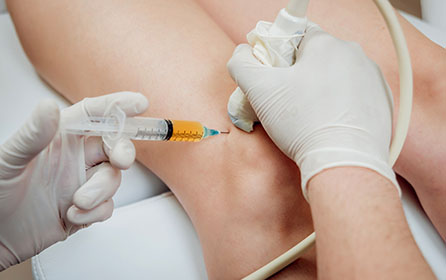
Cortisone/Toradol
Cortisone is a man-made synthetic version of cortisol. When injected locally into joints, for example, cortisone can provide short-term pain relief and reduce swelling and inflammation.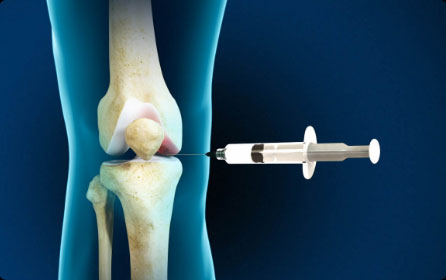
Hyaluronic Acid
Hyaluronic Acid (HA) is a naturally occurring molecule in the body, mostly found in the skin, within eye sockets and other joints and tissue. It is typically injected into the affected area.
Platelet Rich Plasma (PRP)
PRP involves the concentration of the patient’s own GF-producing platelets. It is best for patients under the age of 50. This is because platelet numbers and quality decrease as we age.
Wharton's Jelly/Regenerative Cells
Wharton's jelly is derived from the Growth Factors naturally present in the Umbilical Cord. Donors are screened for any possible infectious diseases prior to donation and only accepted after a healthy, natural birth. 1 CC dose for small joints such as single knee or small joint - $3500 or 2 CC dose for larger joints such as hip or shoulder - $4500. Either injection can be supplemented with a series of HA for an additional $750.Post-treatment
We are really with you, all the way.
Post-treatment, we will conduct regular follow-ups to monitor your healing and may offer other treatments to boost the regenerative process. Typically, you can see results in 6 months or less depending on your age and other factors that are key to recovery.
Not a problem.
There is no obligation to continue with treatment following the complimentary consultation.



